5-Week Trial Designs
EMERGENT-1, -2, and -3 were three pivotal, 5-week, placebo-controlled studies evaluated mean change in PANSS total score in adult patients with schizophrenia experiencing acute psychosis.1,2,4,5
EMERGENT-2 & -3 were phase 3 trials, and EMERGENT-1 was a phase 2 trial.1,2,5
See additional study design details.
52-Week Trial Designs7,9
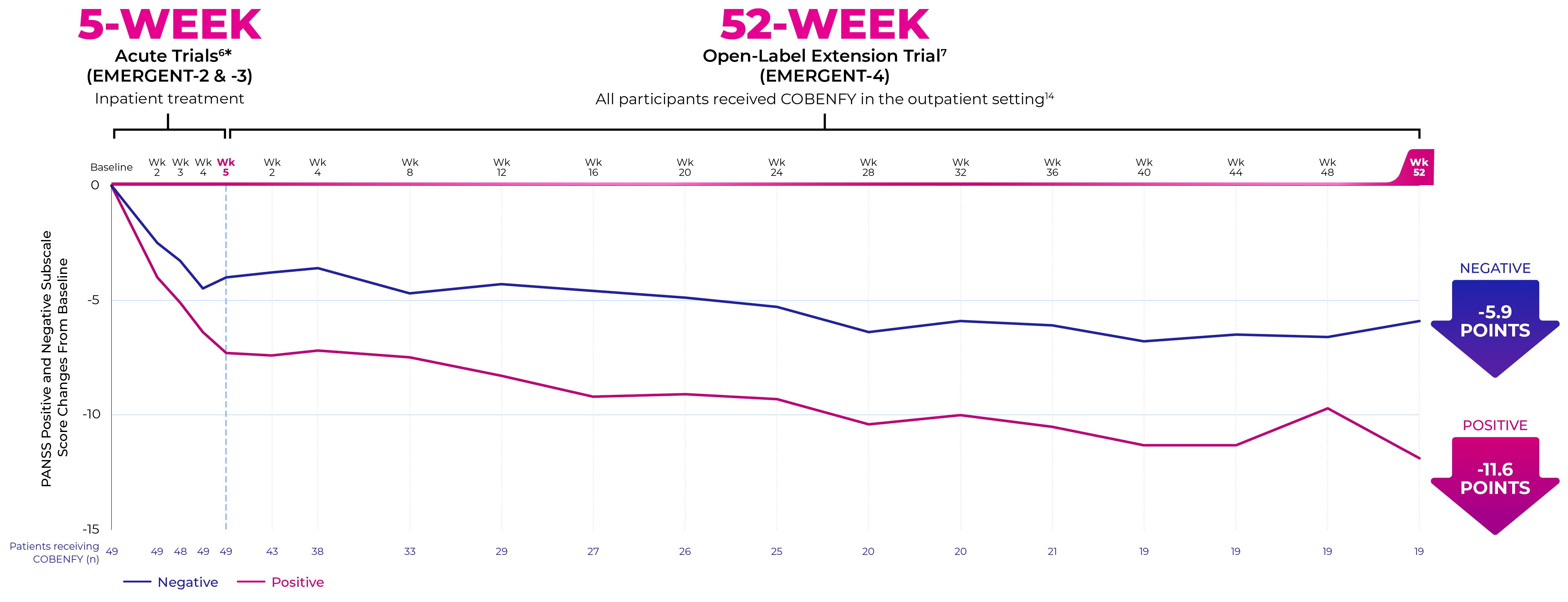
EMERGENT-4 was a 52-week, open-label, extension trial in people who completed the treatment period of the 5-week, randomized, double-blind, placebo-controlled EMERGENT-21 and EMERGENT-32 trials (N=152).7
EMERGENT-5 was a phase 3, multicenter, outpatient, 52-week, open-label trial in adults with a confirmed diagnosis of schizophrenia who have stable symptoms of schizophrenia and have had no prior exposure to xanomeline/trospium; participants with a Positive and Negative Syndrome Scale (PANSS) total score ≤80 and a Clinical Global Impression–Severity (CGI-S) score ≤4 were eligible.9
See additional study design details.